Antiviral Peptide
Preventing Pandemic
Preventing Pandemic
Viruses possess an internal blueprint, such as mRNA, and an external lipid membrane,
making them a type of LNP. LUCA AICell has been conducting research aimed at inhibiting
viral propagation by disrupting LNPs of particles ranging in size from 100 to 200 nm,
which corresponds to the size of most viruses.
To break down LNPs for viruses, LUCA AICell has designed and optimized antiviral peptides.
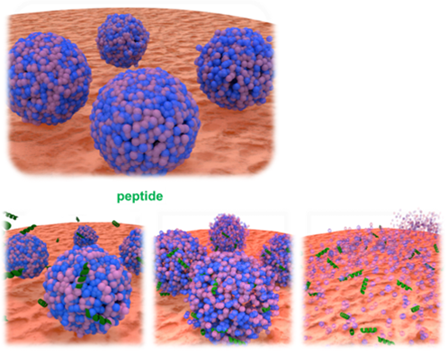
The Lipid Envelope Antiviral Disruption (LEAD) technique is a method that selectively destroys the viral membrane, inhibiting viral infection, without affecting the host (human) cell membrane. This LEAD technique is not limited to a specific virus but can also be applied to unpredictable diseases, such as Disease X.
This research has been accepted by the National Institutes of Health (NIH) as a next-generation virus defense study and is currently being conducted in collaboration with a research team at Stanford University for preclinical research. The primary goal of this research is to validate the effectiveness of LEAD against various viruses, including Covid-19, Zika, and Dengue viruses.
This research is a comprehensive study that requires technologies related to the creation, destruction, and observation of viruses and lipids. The following technologies have been employed:

Virus mimic
This research involves creating LNPs with sizes ranging from 100 to 200 nm, mimicking viruses instead of using actual viruses for experimentation. By manufacturing LNPs of various sizes, it is possible to simulate whether a specific drug can selectively destroy viruses without harming the cells.

Virus tethering
This technology involves developing a platform that allows the observation of viruses. It enables microscopic observation of how the desired virus reacts when exposed to a particular drug.

Peptide optimization
This research focuses on optimizing peptides to efficiently and accurately target and destroy only the desired size of LNPs, as defined in the study.
| Number | Title | Author | Date | Votes | Views |
| 5 |
The race for antiviral drugs to beat COVID — and the next pandemic
Elie Dolgin
|
2023.06.30
|
Votes 1
|
Views 165
|
Elie Dolgin | 2023.06.30 | 1 | 165 |
| 4 |
Chemical Design Principles of Next-Generation Antiviral Surface Coatings.
lucaaicell
|
2023.06.30
|
Votes 0
|
Views 183
|
lucaaicell | 2023.06.30 | 0 | 183 |
| 3 |
Biophysical Measurement Strategies for Antiviral Drug Development: Recent Progress in Virus-Mimetic Platforms Down ...
lucaaicell
|
2023.06.30
|
Votes 0
|
Views 156
|
lucaaicell | 2023.06.30 | 0 | 156 |
| 2 |
Lipid Membrane Interface Viewpoint: From Viral Entry to Antiviral and Vaccine Development
lucaaicell
|
2023.06.12
|
Votes 0
|
Views 165
|
lucaaicell | 2023.06.12 | 0 | 165 |
| 1 |
Role of Membrane Stretch in Adsorption of Antiviral Peptides onto Lipid Membranes and Membrane Pore Formation.
lucaaicell
|
2023.06.12
|
Votes 0
|
Views 178
|
lucaaicell | 2023.06.12 | 0 | 178 |